Lin Hu's group has made important progress in oxazetidines catalyzes asymmetric ring-opening reactions to form chiral ethers
In June 2020, Lin Hu's group published a research paper entitled ‘Ring-Strain-Enabled Catalytic Asymmetric Umpolung C-O Bond-Forming Reactions of 1,2-Oxazetidines for the Synthesis of Functionalized Chiral Ethers’ in Organic Letters.
The ring strain energy of 1,2-oxazetidine is higher than that of other small strained compounds, but there are still few studies on its chemical properties, especially the research on asymmetric catalytic ring-opening reactions.
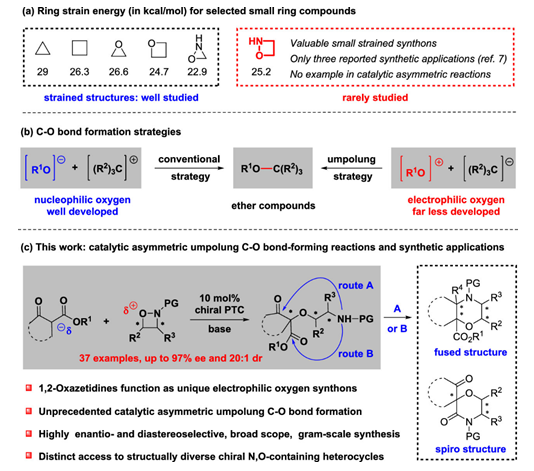
In the presence of a chiral phase-transfer catalyst, Lin Hu's group achieved an unprecedented catalytic asymmetric umpolung C–O bond-forming reaction of N-nosyl 1,2-oxazetidines with β-keto esters, allowing access to a range of highly functionalized chiral ethers bearing quaternary and no adjacent stereogenic centers with high yields, excellent enantioselectivities, and diastereoselectivities (up to 97% ee and 20:1 dr). These versatile products could be flexibly transformed into biologically important chiral fused and spiro morpholines in two steps.
It was found that the asymmetric ring-opening reaction of N-nosyl 1,2-oxazetidines had good compatibility with various substituents in the aromatic ring of the ninhydrin substrate. It was found that chiral ethers with discontinuous chiral centers can be obtained by the reaction of 3-alkyl or 3-aryl substituted chiral N-nosyl 1,2-oxazetidines with indene-2-formic tert-butyl ester with good Dr Value and high yield, even oxazetidines substituted by 4-phenyl group has obtained good reaction results.
The functionalized chiral ether compounds also have high synthetic application value. It can synthesize highly diastereoselective and cyclomorpholine compounds through deprotection of the nosyl group, reduction or nucleophilic addition reactions. It can also be converted by reductive cyclization and oxidation to obtain high enantioselective spiromorpholine compounds.
View Article Online: https://doi.org/10.1021/acs.orglett.0c01916