Zhen Wang's group has made progress in the asymmetric construction of the tricyclic chromanone skeleton
In October 2020, Zhen Wang's group published a research paper entitled ‘Enantioselective Synthesis of Chromanones through Organocatalytic Tandem Reactions’ in Advanced Synthesis & Catalysis.
Asymmetric chromanone derivatives exist in various natural products and have a wide range of physiological activities. At present, the use of asymmetric conversion of substrates containing chromone skeletons is the main method for constructing optically pure chromanone derivatives. Therefore, it is urgent to develop a mild and efficient method to construct chromanone skeleton with multiple chiral centers.
Zhen Wang's group used 1-(2-hydroxyaryl) -1,3-diketones compounds and α,β-unsaturated aldehydes under the catalysis of diarylprolinol silyl ether to provide chromanone products with O,O-acetal fused tricyclic skeleton simply and efficiently through the reaction of Michael addition/ cycloketalization/hemiacetalization (Figure 1). It provides a new idea for the synthesis of natural products with multi-chiral centers and related pharmaceutical chemistry research. The reaction system has the advantages of wide range of substrates, good tolerance of functional groups and mild reaction conditions. The system can also be applied to the synthesis of biologically active glucose and estrone derivatives.
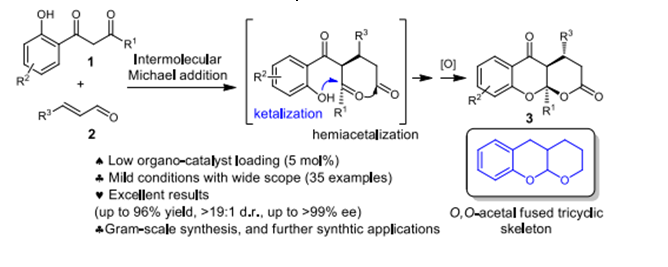
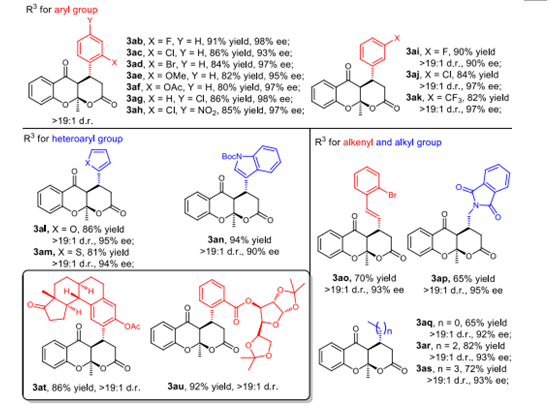
Figure 1. Substrate scope of reaction between α,β-unsaturated aldehydes.
The work was financially supported by the Fundamental Research Funds for the Central Universities (2020CDJQY-A042), venture & Innovation Support Program for Chongqing Overseas Returnees (cx2019063), the Chongqing Research and Frontier Technology (cstc2018jcyjAX0454), the National Natural Science Foundation of China (Nos. 21702185).
View Article Online: https://doi.org/10.1002/adsc.202001031