The Medicinal Chemistry Biology Group of Chongqing University has published an article in Organic Letters entitled " DNA-Compatible Diversification of Indole π-Activated Alcohols via a Direct Dehydrative Coupling Strategy".

DNA-encoded chemical library (DEL) is a new technology that combines the diversity advantages of combinatorial chemistry with the specific encoding of DNA tags, to synthesize ultra-large libraries for rapid selection and discovery of active molecules. It has become a platform for drug discovery widely used in academia and the pharmaceutical industry.
Developing DNA-compatible chemical reactions is one of the core tasks of DEL, but currently reported compatible reactions are insufficient, which greatly limits the chemical spatial and structural diversity of DEL. Therefore, it is necessary to develop DNA-compatible reactions to introduce biologically active molecular scaffolds and construct focused DELs.
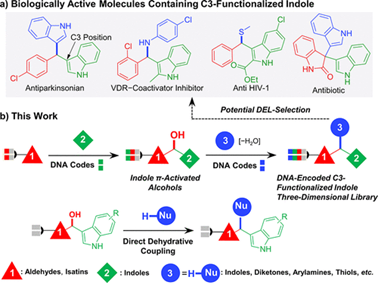
Indole is an important active scaffold and widely exists in drugs and biologically active molecules. In this work, we utilize the high reactivity at the C3 position of indole to construct an indole π-activated alcohol intermediate, which can via a direct dehydration coupling reaction with varieties of nucleophiles to generate C-C/C-N/C-S bonds. We further explored the generation of quaternary alcohol intermediates and constructed quaternary carbon centers through subsequent derivatization reactions. We expanded more than 200 substrates, and verified the feasibility of this strategy for constructing DELs through experiments such as orthogonal reaction and ligation.
In conclusion, we have presented an approach for the highly efficient on-DNA synthesis of diverse C3-functionalized indole derivatives via indole π-activated alcohol. Which can generate three different bonds (C–C/C–N/C–S), and can build a quaternary carbon center to further increase the molecular complexity. This strategy is expected to construct a C3-functionalized indole three-dimensional DEL to facilitate the discovery of hit compound.
The first authors of this article are Zhong Shuting Zhong and Fang Xianfu, students of Chongqing University.