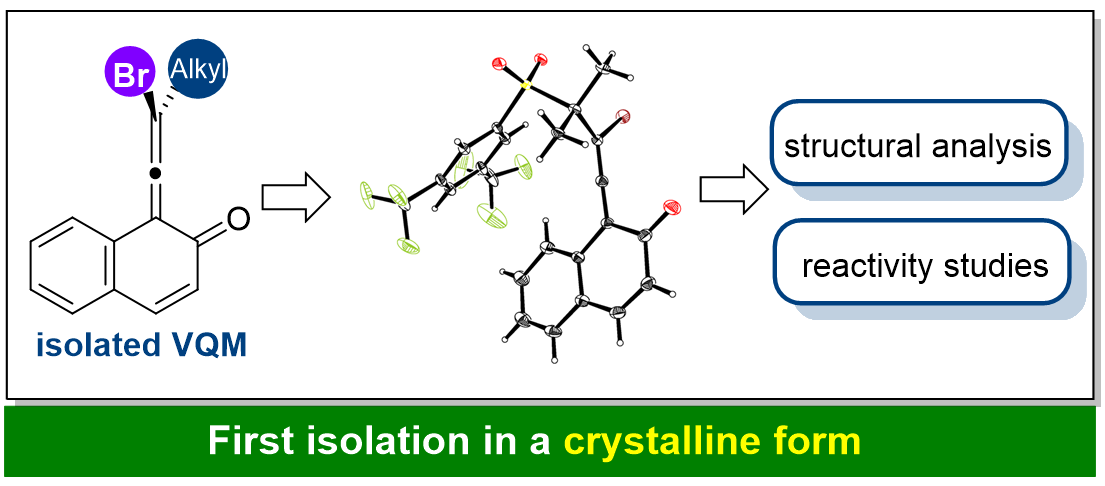
On Feb. 16th, 2022, Professor Yan Hailong group at the School of Pharmaceutical Science of Chongqing University has published an article on Angewandte Chemie International Edition entitled "An Isolable Vinylidene ortho-Quinone Methide: Synthesis, Structure and Reactivity".

Commonly, an elusive intermediate is generated from a precursor and then trapped and consumed in a reaction. Vinylidene ortho-quinone methides (VQMs) have been demonstrated as transient axially chiral intermediates in asymmetric catalysis due to their orthogonal π-bonds forming an allene motif. The current understanding of VQMs is primarily based on time-resolved absorption, trapping experiments and computational studies. Herein, Professor Hailong Yan's group reports the first isolation and comprehensive characterization of a VQM, including crystallographic analysis. The disturbed aromaticity of the VQM led to its high reactivity as an electrophile or a 4π-component capable of asymmetric dearomatization of an electron-deficient phenyl group. Notably, the VQM could be isolated in an enantiomerically enriched form, and the subsequent transformation was stereospecific, indicating that the generation of the VQM was involved in the enantio-determining step. This study paves the way for the direct application of VQMs as starting materials.