On 24 May 2021, Professor Feng Xuli Feng group at the School of Pharmaceutical Science of Chongqing University published a paper on Small entitled “Nucleus-targeted Delivery of Multi-protein Self-assembly for Combined Anticancer Therapy”.
Protein therapy has the potential to revolutionize medicine, but the delivery of multiple proteins is challenging because it requires the development of a strategy that enables different proteins to be combined together and transported not only into cells, but also to the desired cell compartments, such as nucleus.
Here, they present an efficient intranuclear protein delivery nanoplatform based on modified ribonuclease A (RNase A) tuned self-assembly. RNase A bioreversibly modified with adamantane is functionalized with wind chime-like lysine modified cyclodextrin (WLC) to generate RNase A-WLC (R-WLC). R-WLC can not only enhance the cellular uptake of RNase A and accumulate it into the nucleus, but also works as nanovehicles to efficiently transport deoxyribonuclease I (DNase I) into the nucleus, resulting in greatly improved antitumor efficacy in vitro and in vivo.
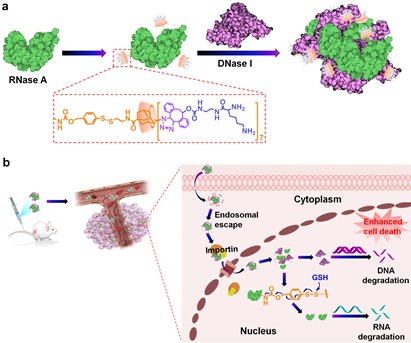
Subsequently, self-assembled protein nanoformulations could significantly enhance the cellular uptake of RNase A and DNase I. Besides, self-assembled protein nanoformulations could significantly enhance the cellular uptake of proteins and transport them to the nucleus, thereby improving their ability to destroy tumor cells for effective cancer treatment. These results collectively suggested that our nucleus-targeted combined protein therapy provides a promising strategy for cancer treatment.
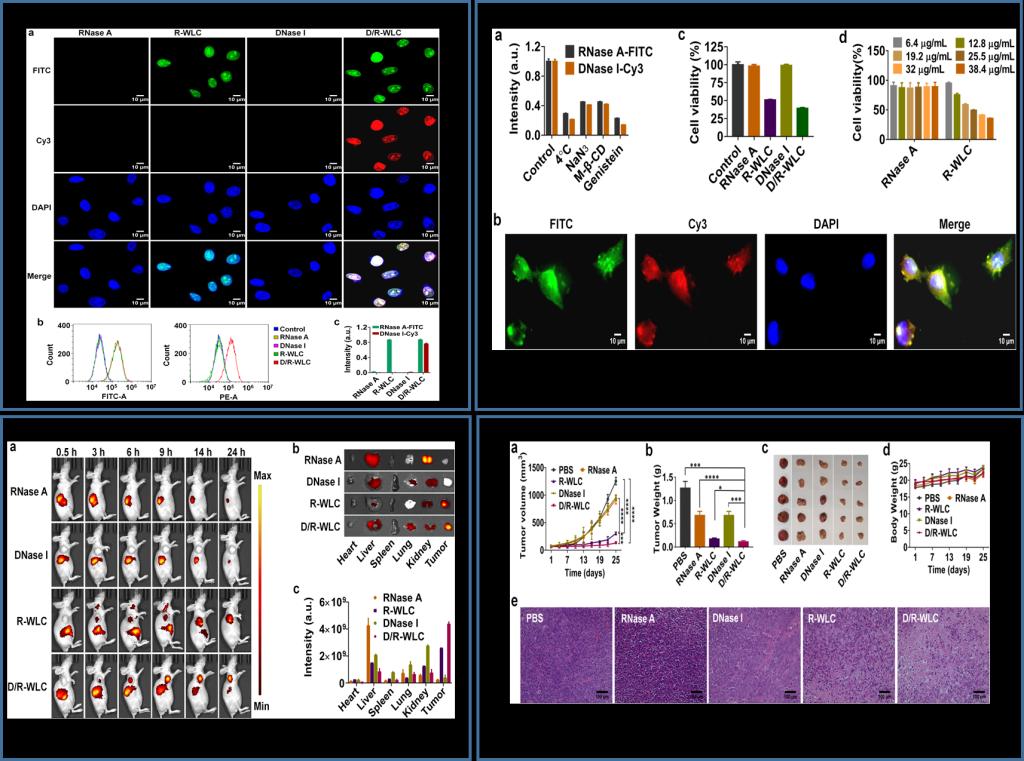
In conclusion, they present a facile and robust nucleus-targeted protein delivery nanoplatform based on protein coordinated self-assembly. In this strategy, WLC functionalized RNase A was used as building blocks to construct well-defined multiple protein nanoparticles through direct self-assembly with DNase I. The self-assembled D/R-WLC nanoparticles showed enhanced cellular uptake and accumulation of both RNase A and DNase I in the nucleus of the same cell, thereby significantly improving their cell damage ability. Moreover, D/R-WLC nanoparticles could selectively accumulate at the tumor site, resulting in greatly improved anti-tumor efficacy of RNase A and DNase I. Therefore, their work demonstrates the great potential of protein assembly as an attractive and powerful tool for developing nucleus-targeted protein delivery nanoplatform for the treatment of various diseases.
The first authors of this article is Tang jiakun, graduate students of Chongqing University. This work was supported by National Natural Science Foundation of China (21773268, 21877010), Fundamental Research Funds for the Central Universities (2019CDYGYB006), Chongqing Graduate Student Research Innovation Project (CYB18056, CYB19067), and the Startup Funding of Chongqing University (0236011104419).
Related link: https: // doi.org/10.1002/smll.202101219