On March 17th, 2023, Professor Zhang Min group at the School of Pharmaceutical Science of Chongqing University published an article on Organic Letters entitled "Arylation of Cyclopropanol with Pyrrole: Asymmetric Synthesis of Indolizidine 167B, Indolizidine 209D, and Monomorine I".

A Fe(NO3)3-mediated ring-opening arylation of cyclopropanol with the electron-rich pyrrole has been developed, which might proceed through oxidative radical ring opening of cyclopropanol followed by cyclization to the pyrrole motif and then aromatization. This method enables direct arylation of cyclopropanol without prefunctionalization and thus allows rapid access to a diverse array of chiral 5,6,7,8-tetrahydroindolizines from easily available chiral amino acid esters. The synthetic utility has been demonstrated by the asymmetric synthesis of alklaoids (−)-in-dolizidine 167B, (+)-indolizidine 209D, (+)-monomorine I, and a natural product analogue.
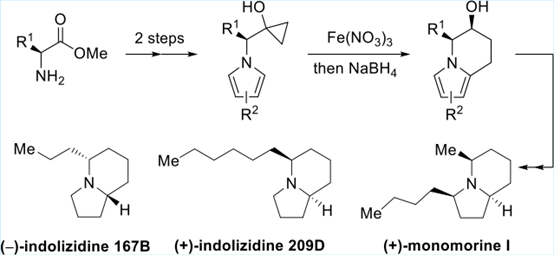
The first authors of this article are Liu Shuangwei and Su Xiaojiao. Zhang Min, He Ling and Qiu Hanyue are corresponding authors of this article.
Related link: https://doi.org/10.1021/acs.orglett.3c00406